
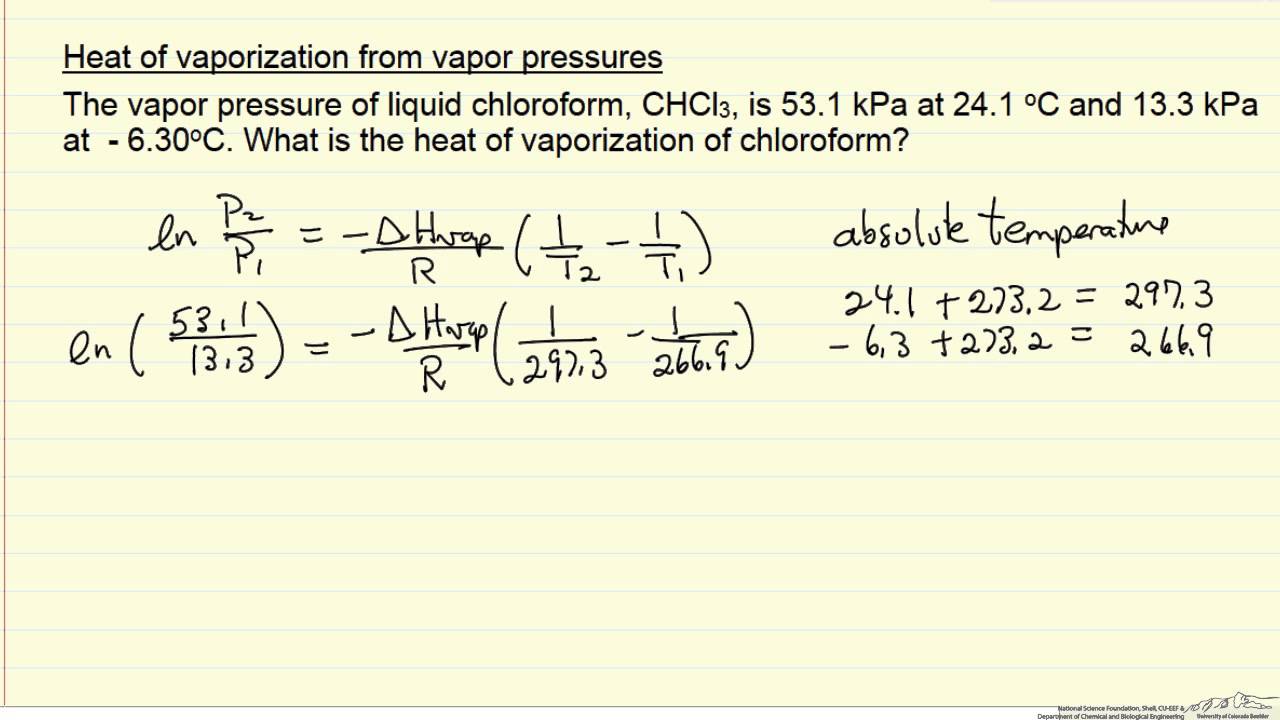
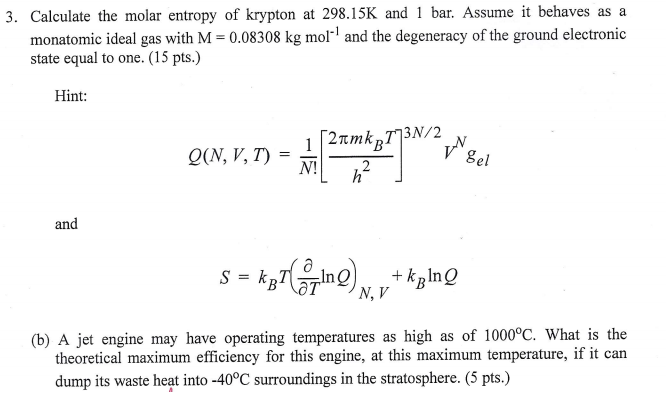

Trouton s rule states that for many liquids at their normal boiling points, the standard molar entropy of vaporization is about 88 I/mol-K. If the molar entropy of vaporization can be treated as a constant, as Trouton s rule suggests, then the relative elevation of the boiling point, AT/T, is universally related to the mole fraction X2 according to Eq. Moreover, recalling van l Hoff s law of osmometry, UV = X2RT, we can relate Eq. Note that the way of derivation here runs via Raoulfs law and the Clausius - Clapeyron equation, whereas ebullioscopy is derived usually via the chemical potential. Equation (6.85) seems to be closely related to the ebullioscopy law and the law emerging in vapor pressure osmometry. Here, ASy is the molar entropy of vaporization. Įthanol (CH3CH2OH) has a normal boiling point of 78.4☌ and a molar enthalpy of vaporization of 38.74 kj mol Calculate the molar entropy of vaporization of ethanol and compare it with the prediction of Trouton s rule. Trouton s rule summarizes this observation. Remarkably, most liquids have similar values for the molar entropy of vaporization. In other words, the boiling point of a liquid, Tbp, is equal to molar enthalpy of vaporization, AHyap, divided by molar entropy of vaporization, ASyap. Because intermolecular forces are not significant in the gaseous state, most substances have similar values for molar entropy of vaporization, ASy p. Table 5 lists the molar enthalpies and entropies of fusion and vaporization for some elements and compounds. Like water, almost all substances can be in the three common states of matter. If heat-of-vaporization data are not available, the molar entropy of vaporization for nonpolar liquids can be estimated via an empirical equation of Kistyakowsky. Related Calculations, This procedure can be used to obtain the entropy of phase change for any compound. Ĭalculate the molar entropy of vaporization for liquid hydrogen iodide at its boiling point. Calculate the molar entropy of vaporization at its normal boiling point for each of the following (a) molecular oxygen (b) ethane (c) benzene and (d) mercury. Table lists molar enthalpies of vaporization of several substances. The Trouton constant is the molar entropy of vaporization. Associated liquids show marked variations from this value. Por most normal liquids the constant has a value of approx.


 0 kommentar(er)
0 kommentar(er)
